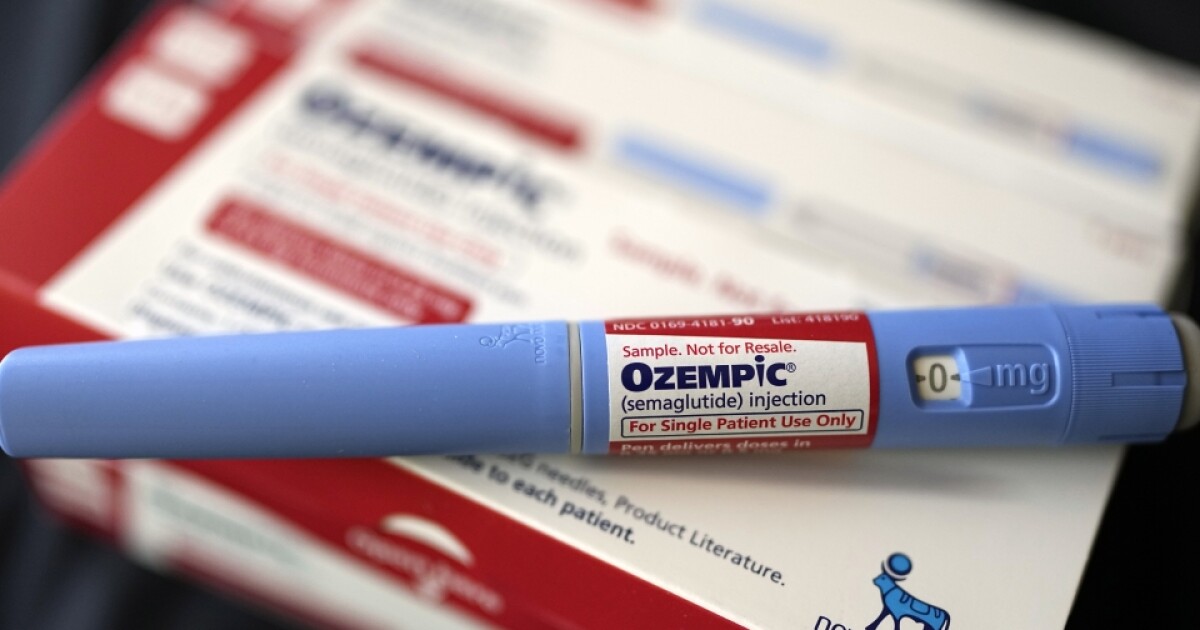
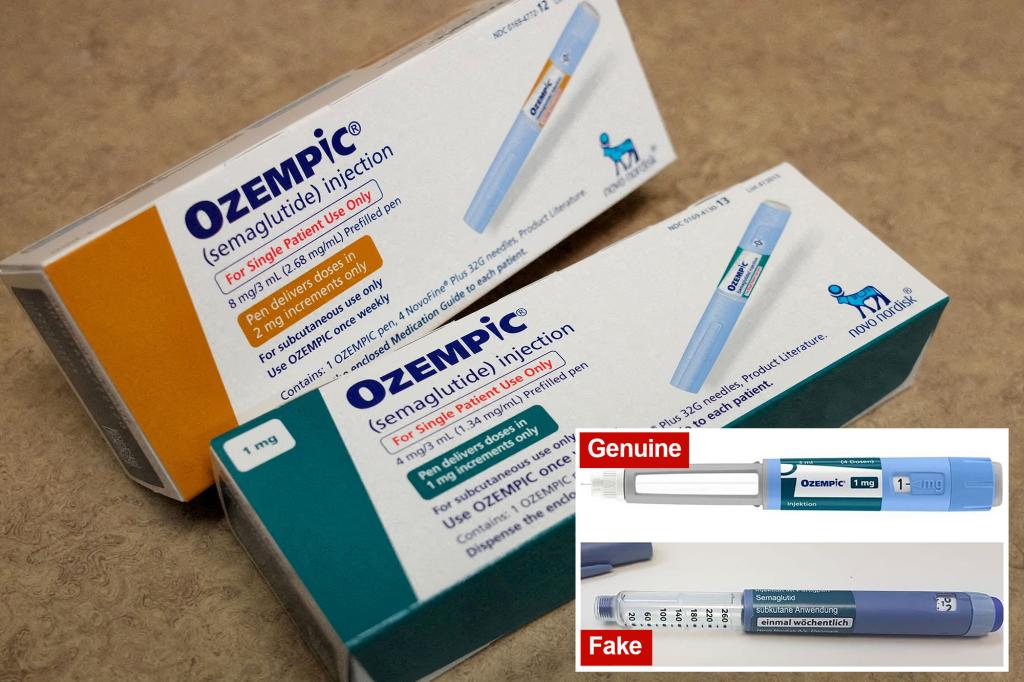
Fake Ozempic pens contain insulin, worrying health officials
TAMPA, Florida. You have probably already heard of the drug Ozempic, used to treat diabetes. But along the way, doctors discovered that it also helped patients lose weight by reducing their cravings. This drug, in high demand, is causing supply shortages around the world. Now, there’s a new warning from… Continue Reading Fake Ozempic pens contain insulin, worrying health officials