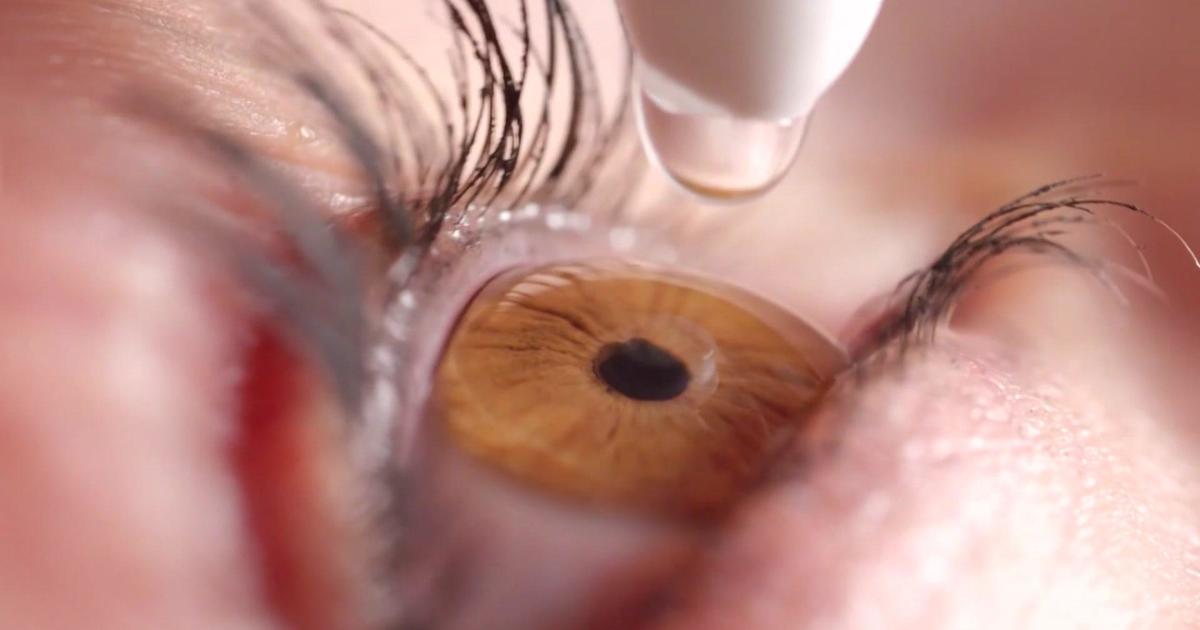
Yes, the FDA warns against using eye drops from multiple brands due to the risk of infection.
Many people use eye drops to treat certain eye problems, such as allergies, dryness, irritation, and redness. Recent online search trends show that some people are wondering whether the Food and Drug Administration (FDA) has issued a new warning regarding eye drops sold in the United States. THE QUESTION Is… Continue Reading Yes, the FDA warns against using eye drops from multiple brands due to the risk of infection.