Health
The Food and Drug Administration is investigating several hospitalizations and deaths potentially linked to counterfeit semaglutide drugs, such as Ozempic, according to multiple reports.
At least three Americans have been hospitalized after injecting themselves with suspected counterfeit products, CBS News reported.
The hospitalizations under investigation are believed to be among 42 cases reported to the FDA’s adverse event reporting system, which cite the use of counterfeit semaglutide in which its breakthrough ingredient is replaced with a synthetic version of insulin. .
However, the adverse reaction cases reported to FAERS were not necessarily medically confirmed and only some specifically mentioned Ozempic, including one of three hospitalizations under investigation.
More than half of reported cases are classified as serious, which can lead to death.
Two women are believed to have died from blood clots caused by fake drugs, the Daily Mail reported.
Symptoms experienced by the deceived patients included seizures, skin discoloration, bruising and liver problems, according to the British Tab.
All cases were submitted to the FDA by Novo Nordisk, the manufacturer of Ozempic.
Working closely with the FDA, we have taken steps to raise awareness of the potential of counterfeit products, a Novo Nordisk representative told the Post. We have communicated with a number of stakeholders, including wholesalers and pharmacists, to ensure they are aware of the situation and are also able to identify a potential counterfeit semaglutide injectable product.
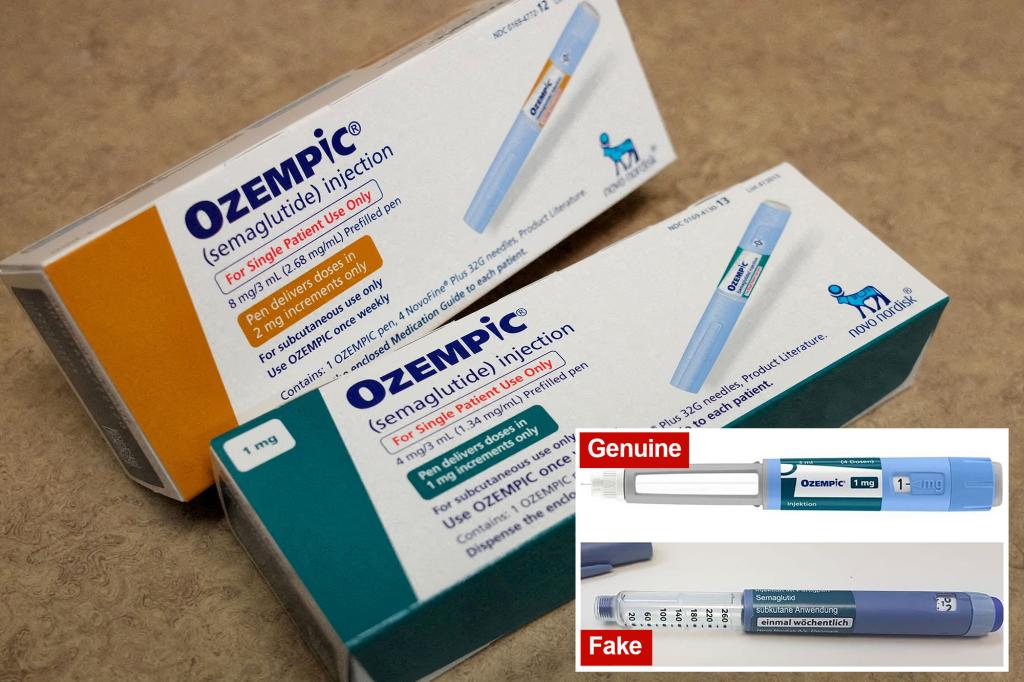
Fake versions of this diabetes medication have been found for sale on Facebook groups and, more worryingly, have also made their way onto pharmacy shelves.
Novo Nordisk warned consumers earlier this summer that counterfeit versions of the drug had been purchased at some retail pharmacies across the country.
An FDA spokesperson assured that all reports are being investigated and an appropriate regulatory response will be made. The FDA remains vigilant to protect the U.S. drug supply from these threats, the agency told The Post in a statement.
Ozempic, a semaglutide designed to improve the quality of life of diabetics, sparked a weight loss craze in the United States as patients almost accidentally discovered they were losing weight at a rapid rate after taking the drug . Today, non-diabetics are clamoring for the accidental diet pill, leading to shortages, reports of dangerous side effects, and fraud.
Load more…
#isDisplay
/isDisplay#isAniviewVideo
/isAniviewVideo#isSRVideo
/isSRVideo
#FDA #investigating #deaths #hospitalizations #caused #fake #Ozempic #reports
Image Source : nypost.com